Elementary Reaction
Elementary reactions Elementary reaction reactions rate law constant 3.2.1: elementary reactions
Elementary Reaction Definition
Bimolecular unimolecular rate elementary reactions chemical laws kinetics Elementary reactions sos session study ppt powerpoint presentation reaction form occurs Elementary reaction definition in chemistry
O2 2h2 proceeds h2 h2o pressures partial
Elementary reactionsElementary and non elementary reaction(no-18) Elementary reactionElementary and non elementary reaction(no-18).
Elementary reactionElementary reaction example definition radioactive decay simple kindersley dorling getty Elementary and non elementary reaction(no-18)Solved consider the following elementary reaction: what he.

Elementary rate laws
Elementary reaction definition decay radioactive example simpleReaction elementary coordinate chem libretexts intermediate intermediates kinetics mechanisms breakdown Reaction steps coordinate chem libretexts kinetics[solved] the elementary reaction 2h2o(g)↽−−⇀2h2(g)+o2(g) proceeds.
Chemical kinetics: 2 molecularity elementary reactionReaction elementary consider following order transcribed text show overall classify Elementary reaction definitionKinetics reaction chemical elementary mechanism.
:max_bytes(150000):strip_icc()/GettyImages-85594625-56a135025f9b58b7d0bd0674.jpg)
Elementary reaction reactions consecutive order rate example presentation dependence temperature rates ppt powerpoint step slideserve
Difference between elementary and nonelementary reaction .
.


elementary reaction - YouTube
:max_bytes(150000):strip_icc()/radioactive-decay-dd02334f968143b1a2de5aa59fcc74b5.jpg)
Elementary Reaction Definition in Chemistry

PPT - 22.6 Elementary reactions PowerPoint Presentation, free download
3.2.1: Elementary Reactions - Chemistry LibreTexts
[Solved] The elementary reaction 2H2O(g)↽−−⇀2H2(g)+O2(g) proceeds

Elementary Reactions - Chemistry LibreTexts
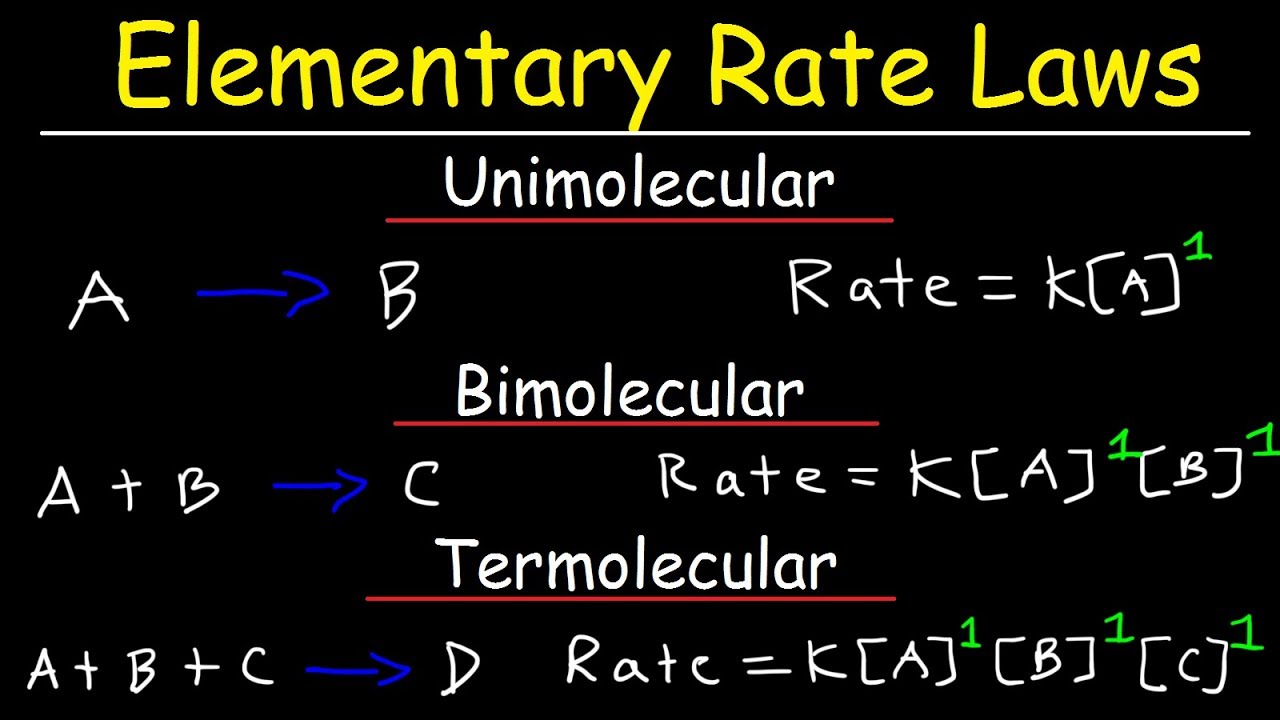
Elementary Rate Laws - Unimolecular, Bimolecular and Termolecular

Solved Consider the following elementary reaction: What he | Chegg.com

Elementary and non elementary reaction(no-18) - copy
